Abstract
Background: Venous thromboembolism (VTE) is rare in patients younger than 21 years of age. The differences in clinical features between pediatric, adolescent, and young adult VTE patients have not been well defined.
Aims: To compare clinical characteristics at baseline, risk factors, management and outcomes between pediatric, adolescent and young adult patients with VTE.
Methods: Data from the prospective international RIETE registry were used. Patients younger than 21 years of age with all types of objectively confirmed VTE were stratified into three sub-cohorts: children up to 12 years old, adolescents 12 - 18 years, and young adults 18 - 21 years. Adolescents and young adults were also compared with patients older than 21 years.
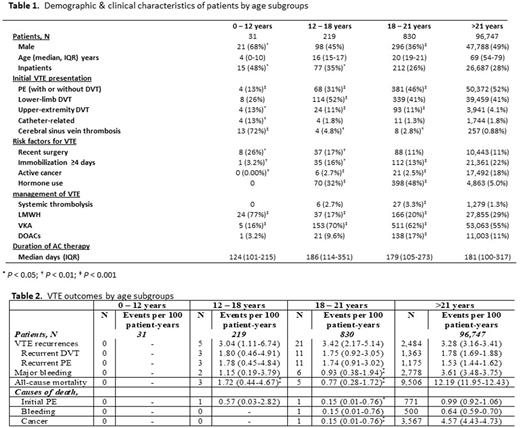
Results: Of the 97,827 RIETE patients enrolled until March 2022, 31 were children, 219 adolescents and 830 young adults (median [IQR] age 4 [0-10], 16 [15-17], 20 [19-21] years, respectively). Most younger patients were males (P < 0.001), with increasing proportion of females once hormone use became a risk factor (after the age of 12, P < 0.001). Children presented with less pulmonary emboli and more cerebral sinus vein thrombosis and catheter related thrombosis (P < 0.01). Most pediatric patients were treated with LMWH (Table 1).
Rates of VTE recurrence, major bleeding and all-cause mortality were comparably low among adolescents and young adults. None of these occurred in children younger than 12 years. None of the patients in any of the pediatric sub-cohort died from VTE recurrence (Table 2).
Conclusions: Although often grouped together, children, adolescents and young adults have distinctive VTE features. While VTE presentation may be similar among adolescents and young adults as compared to older patients, the outcomes of patients younger than 21 years are more favorable.
Disclosures
Cohen:Pfizer: Research Funding. Barg:Pfizer, Roche, NovoNordisk: Honoraria. Monreal:Leo Pharm: Research Funding; Rovi: Research Funding; Sanofi: Membership on an entity's Board of Directors or advisory committees, Other: Presented to congress, Research Funding. Kenet:Opko Biologics: Consultancy, Research Funding; Shire: Research Funding; Alnylam: Research Funding; BPL: Consultancy, Research Funding; ASC Therapeutics: Consultancy; Novo Nordisk: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Roche: Consultancy, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Bayer: Consultancy, Honoraria, Research Funding; UniQure, SPark, Sobi, CSL: Honoraria; Sanofi-Genzyme: Consultancy, Honoraria; BioMarin Pharmaceutical Inc.: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal